Large Scale CF survey on Personal Health and Wellness
The Cystic Fibrosis (CF) community is ripe for a new model of patient engagement in research, with medical data collection and sharing, through new technologies that ensure privacy and allow for patient compensation. [1]The CF community is one of the most unified patient and patient advocate groups, with decades of experience and knowledge sharing. [2] The genetics of cystic fibrosis, while complex, is tractable for targeted drug therapies, and the past decade has witnessed breakthrough treatments. [3] The CF Foundation has been instrumental in supporting the basic research, compound discovery, and drug development that led directly to two billion-dollar blockbuster drugs: ivacaftor and lumacaftor, developed by Vertex Pharmaceuticals. These two drugs can extend life in patients by 10-20 years, yet, even for those with the approved mutations, more than 30% of those eligible cannot access this therapy in the U.S. The numbers are considerably worse outside of the U.S. There is, therefore, a serious unmet need to provide all patients with access to these current therapies as well as drugs that are developed in the future. Furthermore, the high cost and limited access to these drugs has stymied broader research into how other CF patients (i.e. those without the canonical mutations) may benefit from these drugs— and this inaction is despite evidence that the potential number of patients who could benefit is large. [4]
CysticFibrosis.com/cftechnology.org/ is a non-profit, online community with 18,000 U.S. cystic fibrosis patients and caregivers as members. We recently launched a survey, and over 1200 patients and caregivers responded with valuable details about their data collecting behavior and their attitudes towards a new model of patient engagement with researchers and the pharmaceutical industry.
The key points of the survey are:
- Currently, many CF patients and caregivers are either not collecting their personal health data (30%), or are collecting it through paper or hardcopy (50%). Only 16% use apps to store their personal health data.
- CF patients and caregivers are willing and able to collect personal health data (X% patients; X% caregivers).
- CF patients and caregivers want to share this data to help individuals and the community, if they are fairly compensated (X% patients; X% caregivers).
- There is a commitment on the part of the CF community and us to pilot a new approach to the research and development into rare disease treatment.
We propose that the CF community is an untapped resource for richly phenotyped data that is of enormous value to the pharmaceutical industry. CFTechnology.org can test a new paradigm in which participants are compensated for their valuable data by, for example, having the costs of the medicines covered.
To demonstrate the value of the CF community to drug research and development and to build a virtuous cycle in which the participants supplying the data are compensated for their data, we have designed a Pilot Study where data is kept privately by the patients then de-identified when they decide to share it through a mobile app. The participant maintains data ownership and decides when and what information to share.
Background: Improvements in treatment of Cystic Fibrosis have transformed patients’ quality of life and that of their caregivers. These improvements have come from several disciplines: advances in targeted therapeutics, improvements in nutrition and exercise, and patient-driven efforts to share knowledge and insight from individual experiences. Unfortunately, these advances are “lumpy,” that is to say they are not uniformly disseminated across the CF community. Furthermore, the infrastructure for providing, peer-reviewed, patient approved information is rudimentary and fragmented across disparate groups. As a necessary step to build support for the CF community, we undertook a survey of over 1000 CF patients and caregivers to assess their familiarity with their health data, their willingness to collect and share this data, and their interest in taking an active part in the research applications of their data.
Objective: To explore if CF patients and caregivers are willing to collect and share diverse health and behavioral data using mobile applications.
Methods: An anonymous survey of over 1100 CF patients and caregivers using a 15-question online survey.
Results: We found a high level of data tracking across all areas surveyed. Paper/hard copy was the most popular form of medical data tracking and storage, suggesting that the opportunities for digital data tracking are far from saturated. The under 35-year-old demographic was much more likely to respond that they are willing to collect and share data digitally. Surprisingly, less than half of the respondents had genetic test data, representing an opportunity for genomic education. About half of all participants would use an app if it sells their data but gives them remuneration. Both patients and caregivers are willing to share data to advance research.
Discussion and Conclusions: The response rate for our survey was extremely high, with 1100 respondents, close to equally divided between patients and caregivers. The aggregated responses confirmed that the CF community is actively interested in data collection and, where appropriate, sharing this information to drive the research enterprise forward. In the following pages, we summarize these observations and propose a framework in which the CF community can accelerate drug discovery and development and set a new paradigm for revenue sharing.
Introduction
There are several cystic fibrosis organizations in North America and worldwide. In the research area, groups span the spectrum from industry-funded efforts, public-private partnerships, sponsored research in academic labs to basic academic research. In parallel, diverse patient advocacy groups include large philanthropic organizations in North America, the United Kingdom, South Africa, Israel and more countries. As is the case with other rare diseases, multiple patient organizations exist and these groups do not necessarily share the same goals.
The motivation behind CFTechnology.org and our health data survey is to better understand the attitudes and behaviors around personal health data of the CF patients and caregivers in order to determine their ability to participate in drug discovery and development efforts. Because of its position as a “common” rare disease, we believe that cystic fibrosis can serve as an exemplar for other rare diseases.
The cystic fibrosis community is unique: highly educated and highly engaged. The prognosis of individual patients is determined by a combination of factors: genetic, environmental, adherence and unknown. The complex treatment regimen forces patients and caregivers to develop expertise and organization beyond many other diseases. Thus, education and participation in care are crucial. [5]
During the past seven years, the CF community has witnessed the introduction of an entirely new class of drugs: the genetic potentiators, correctors, and combinations of these two. The connection between cystic fibrosis and a patient’s underlying genetics is crucial and something we hope to expand upon. These drugs, while specifically targeted to certain genotypes, have been surprisingly effective on a broader group of patients.[6] In an effort to assess the degree to which this community was familiar with personal data collection and with their willingness to share different data types, we performed a survey of over 1200 patients and caregivers, asking 15 questions that fell into two broad categories;
- What type of data do you collect, and how?
- Are you interested in sharing this data, and on what terms?
Results: Survey Observations
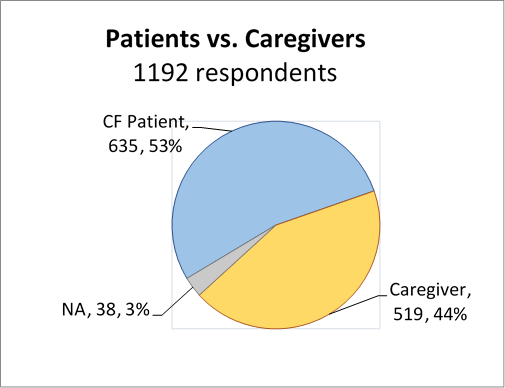
We found close to a 50::50 patient and caregiver participation.
Note: We need to confirm if our data mirrors global population statistics. Also, we need to be able to articulate the role of “digital natives”, i.e. individuals who were raised in the era of broad internet access, in the future of cystic fibrosis advocacy.
An interesting observation from this study is that half of the respondents reported that they know their mutations, and yet their understanding about these mutations and how they affect the manifestation of CF was surprisingly low. This represents an opportunity for education. [7]
There is a gap between the bench (i.e. research advances) and the bedside (i.e. patient quality of life).
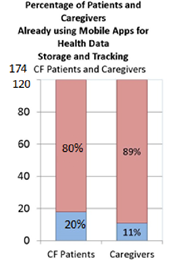
We next asked about health-data tracking behavior, and were surprised to find that a slight majority collected by paper or hard copy.[8] The only significant difference between patients and caregivers in data collection methods was that patients were almost twice as likely to use apps.
Specifically, when one filters the survey data further this is the result:
Patients (Caregivers)
- I don’t keep records 29% (32%)
- paper or hard copy 47% (53%)
- digital files 35% (27%)
- mobile apps 20% (11%)
However, by letting people select as many as apply, the survey did not actually answer the question
‘what method do you use the most or do you prefer’ but rather, ‘list all the methods you use.’ The reliance on old-school paper records was somewhat of a surprise and represents an opportunity for app developers. One might not be surprised to learn that caregivers rely more heavily (vs patients) on paper records. An interesting comparator would be to look for data amongst the ‘quantified-self’ community and ask if our results reflect highly digitized individuals. [9]
We also asked about health tracking behavior and the concerns surrounding data collection. This is admittedly, a leading question, yet the answers are still instructive. It is interesting to note that 17% of the ‘YES I KNOW MY MUTATIONS’ group uses apps to collect health data, and only 10% of the ‘NO, I DON’T KNOW MY MUTATIONS’ group uses apps.
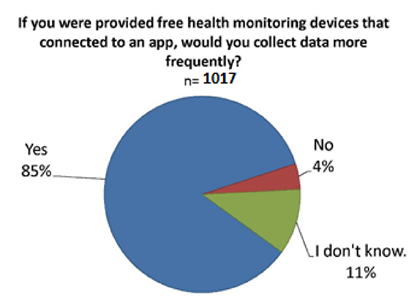
While only 16% of all survey respondents currently use mobile apps to track their health data, 86% said they would be more likely to track health data on an app via a device given to them free of cost that uploads the data to the app. [10]
There were no significant differences between CF patients and caregivers in terms of the types of health data they would be willing to track (examples include medication use, genetic tests, spirometry tests, lab cultures and more). This is encouraging because successful engagement with the CF community will require caregivers and patients, and these data illustrate how unified and energized the community is.
We queried the patients and caregivers about their interest in precision medicine, specifically how do they value their data and what do they consider its value? Surprisingly the estimated monetary value was quite low, with the majority thinking their data was worth hundreds or thousands of dollars. [11]
Specifically, the responses were as follows:
YES, I KNOW MY MUTATIONS (482 respondents)
NO, I DON’T KNOW MY MUTATIONS (89 respondents)
The Yes group thinks their data has the same value as overall survey population does.
The No group actually value their data higher than the respondents overall. There was a big increase in the response indicating that patients thought their data was worth $1000s dollars.
We asked if patients and caregivers were willing to share cystic fibrosis health data to advance research. 67% said yes if they were compensated. The most surprising finding was that 33% of the respondents reported that they don’t need compensation to share their data. This community seems to recognize the value in the collection of data for the advancement of research to benefit the collective group.
When asked “Are you interested in the business model of deciding when and how to share data?” 90% said yes. The aspect of control and permission as important to this group as that of profit or remuneration.
When we analyzed the responses of the subset of patients and caregiver unwilling to share data, we learned that they were less motivated by the possibility of remuneration for data, and more concerned about the privacy policy of the apps. This suggests that non-sharers can potentially be converted if privacy and ownership of their data can be assured.
When we asked if you were to use an app for health data collection, what concerns would you have? Caregivers were slightly more likely to skip this question and they also seemed slightly, but not significantly, more concerned about privacy and transparency.[12] This may be due to age, though we don’t have that data to break down, because we asked the age of the patient they care for, and also this may be due to hesitation to speak/answer for someone else.
We obtained close to 50:50 CF Patient vs. Caregiver participation in the survey as well as a very high overall response rate, with additional participation from medical professionals and non-caregivers.
The age of the CF Patients participating in the survey or represented by a caregiver in the survey is concentrated in the 19-29- and 30-44-year ranges. There is a drop off after 45 years and especially after 59 years, which reflects the life expectancy of the disease. [13]
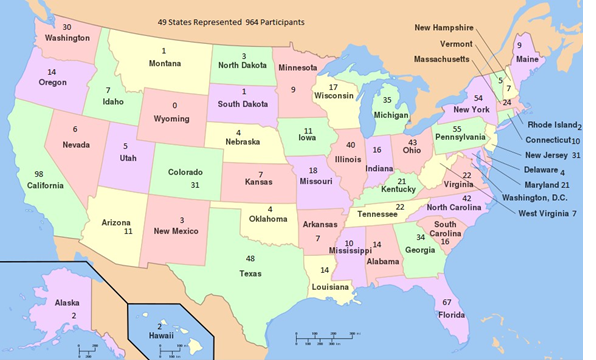
The vast majority of survey participants are from the U.S. but 30 other countries are represented by 136 people.
The countries are: Other Countries: Denmark, Germany, Italy, New Zealand, Pakistan, Barbados, Mexico, Netherlands, Russian Federation, Saudi Arabia, Spain, Sweden, Afghanistan, Belarus, Brazil, Bulgaria, Finland, France, Georgia, Iceland, India, Malta, Peru, Portugal, Romania
The representation by state is somewhat in line with state population size: California, New York, Florida, Pennsylvania, and Texas are in the top five. Illinois is somewhat underrepresented relative to its population size, and North Carolina over represented.
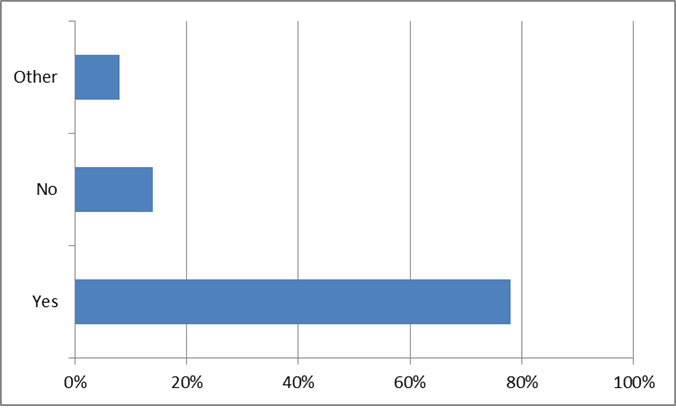
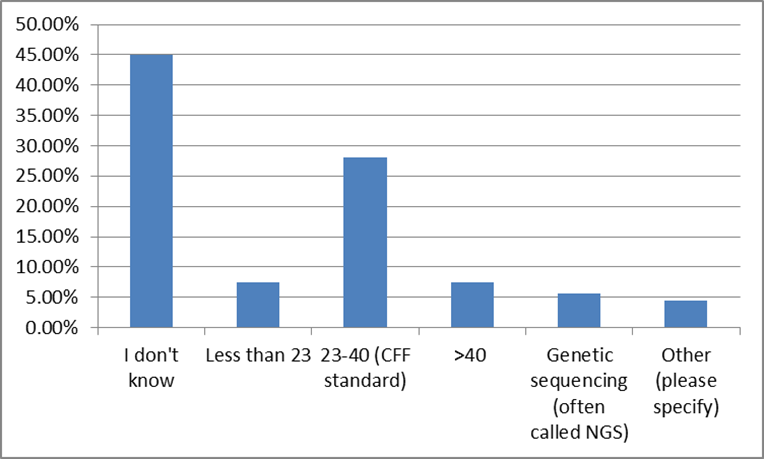
41% of survey participants responded that they know their CF genetic mutations, but a relatively large number of survey participants chose to skip this question. However, when asked about genetic CF testing, only 5% skipped the question. Close to half of the survey participants do not know how many CF mutations they were screened for, and only 8% had full genetic sequencing. [14]
This is an educational opportunity for the CF community. Specifically, these responses suggest that nearly 60% of the community do not know their mutations. Accordingly, by educating those who do not know their mutations as to the value of this information (for them, and the community) we can expand the knowledge base. In this same vein, those who have been tested, but are either unaware of their specific mutations, or unsure of their significance, the opportunity exists to increase understanding. [15] Furthermore, by increasing the overall genetic literacy of the community, the value of this information to pharmaceutical and biotech research increases.[16]
DO YOU KNOW YOUR MUTATIONS?
HOW MANY MUTATIONS WERE YOU SCREENED FOR?
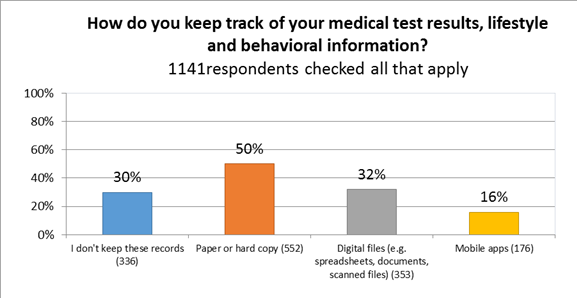
Paper/hard copy is the most common form of medical data tracking and storage.[17] Only 48% of survey respondents checked off a digital option, such as apps or scanned documents and spreadsheets. This is an opportunity for mobile health.
There were 130 comments in response to this question, and many commenters listed the app they use, [18] in most cases only one app per person.
Some comments made by survey respondents:
“If you’re seriously implying that’s what you do, that would be amazing! None others do this that I am aware of, though I never extensively searched.”
“Private industry is going to benefit financially,[19] the potential wealth in genomic data is beyond comprehension.”[20]
“This is an exciting concept to open communication between the actual creators/scientists and patients without using the doctors as translators.[21] They have enough work keeping us alive, we can’t always expect them to ask all the right questions and pass on helpful info. As much research and care CF doctors and scientists do, they don’t wake up every morning praying that the first breath they take won’t be their last, then work all day to shatter the ‘pred’ value limits. I think this will open a new world of info and products to make our lives healthier and easier. THANKS!”
To gauge perceptions regarding data sharing and compensation, we asked the following questions
Sentiment was strongest around wanting to know if an app company sells a person’s health data, and planning to stop using an app if it sells data without a person’s permission. About half of all participants would use an app if it sells their data but gives them remuneration. [22]
Consistent with the openness of this population to data sharing and technology adoption is the
emphasis on tracking/collecting readily actionable, concrete data (e.g. medications, lab cultures genetic test) vs more global info.
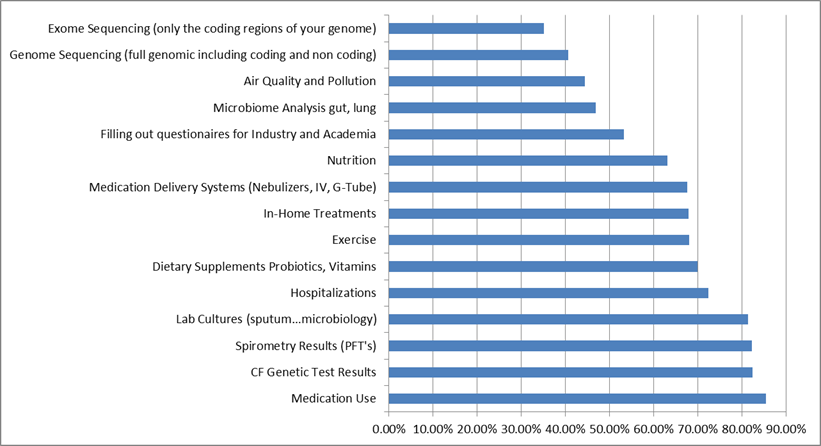
This graph is based on 499 participants. Relatively more Caregivers skipped this question. Comparatively speaking, Caregivers are collecting or willing to collect in more of these data categories (greater in 11 vs. 4 categories).
Not surprisingly, the willingness to collect data tracks with understanding/familiarity. This observation is a strong argument for continual education of new data types and the reasons for collecting such data.
Consistent with previous slide, when queried regarding monetary value of their data,
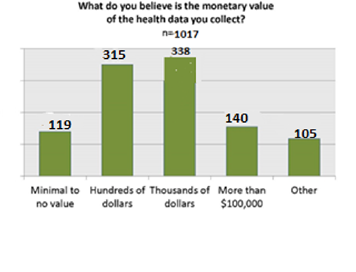
34 believe data is worth $100s dollars or less,[23] yet on next slide, 96% of respondents said they are willing to share data to advance research. This is evidence of a strong altruistic element in the CF community.
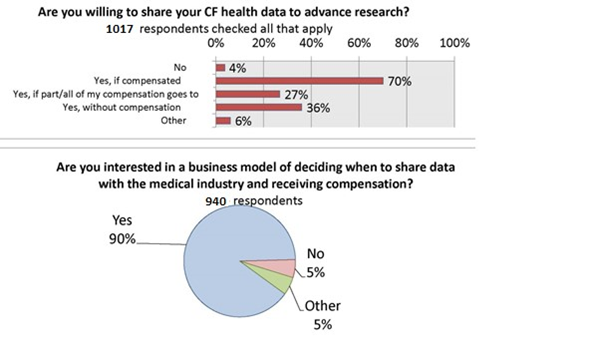
We found that non-sharers are less motivated by the possibility of remuneration for data, and more concerned about the privacy policy of apps. [24] This points to the need for education and discussion regarding privacy concerns within the CF community. [25]
Yet, it does not seem like there is a statistically significant difference, except for the remuneration question.
Ages of CF patients using Mobile apps
6-12 9
13-18 26
13-18 26
19-29 52
30-44 45
45-59 21
60-69 1
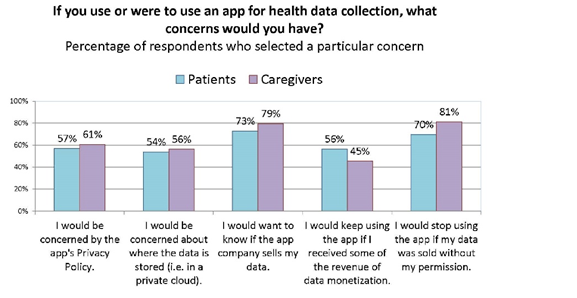
Caregivers were more likely to skip this question. They are also more concerned about privacy and transparency and less motivated by remuneration.
Summary and Perspectives:
The Cystic Fibrosis community of patients, their caregivers and their advocates has been at the forefront of patient empowerment for more than three decades. This community has empowered research by taking it upon themselves to collect their own data, and share it. This patient-first approach to data sharing has directly contributed to some of the most dramatic and innovative new medicines ever developed, including ivacaftor (Kalydeco®), lumacaftor/ivacaftor (Orkambi ®), and tezacaftor/ivacaftor (Symdeko®). As genomic and other ‘omic’ technologies become more commonplace, the amount and quality of CF patient data stands to increase by orders of magnitude, and by combining these new data types with a more formal approach to other phenotypic and lifestyle data (enabled by mobile apps, [26] and protected by Blockchain technologies) the future of a true ‘patient-empowered’ revolution in CF health and wellness is possible.
[1] Rachel Z. Arndt, ModernHealthcare.com(Crain Communications, Inc, 11/30/20, new-platform lets-patients-sell-their-health-data
[2] Barnett, Jeanne, CysticFibrosis.com, (1996), homepage.
[3] Cristensen, Kelsey, KVAL.com NEWS, (May 6, 2019)
https://kval.com/news/local/groundbreaking-research-for-cystic-fibrosis-on-brink-of-finding- universal-treatment
[4] Yasinski, Emma, CysticFibrosisNewsToday.com, (September 24, 2018) vertex-and-treating-cf-costs-can-unsettle-but-vertex-remains-close-to-community-interview-series/
[5] Gathercole K., PubMed, (December 28, 2018), https://www.ncbi.nlm.nih.gov/pubmed/30590956
[6] Van Goor F, Yu H, Burton B, Hoffman BJ, PubMed, (January, 2014), https://www.ncbi.nlm.nih.gov/pubmed/23891399
[7] Fraser-Pitt, Douglas, O’Neil, Deborah, PubMed, (September, 2015), “Cystic Fibrosis- a Multiorgan Protein Misfolding Disease”, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5137970/
[8] Heath, Sara, Patient Engagement, (June 2016), https://patientengagementhit.com/features/how-patient-health-data-access-drives-patient-engagement
[9] Kenton, Will, Quantified Self, (September 2018), https://www.investopedia.com/terms/q/quantified-self.asp
[10] Petrow, Steven, Washington Post, (Nov 2018), “Who owns your medical data? Most likely, not you”, https://www.washingtonpost.com/national/health-science/who-owns-your-medical-data-most-likely-not-you/2018/11/23/28785efc-e77d-11e8-a939-9469f1166f9d_story.html?noredirect=on&utm_term=.66615e63f42b
[11] Thielman, Sam, New York, (January, 2017),“Your private medical data is for sale and it’s driving business worth billions”, https://www.theguardian.com/technology/2017/jan/10/medical-data-multibillion-dollar-business-report-warns
[12]Lord, Nate, DigitalGuardian.com, (June 2018),“Healthcare Security, Understanding HIPPA Compliance, and Its Role in Patient Data Protection”, https://digitalguardian.com/blog/healthcare-security-understanding-hipaa-compliance-and-its-role-patient-data-protection
[13] Editor, CysticFibrosisNewsToday, “Life Expectancy”, https://cysticfibrosisnewstoday.com/cystic-fibrosis-life-expectancy/
[14] Lord, Nate, DigitalGuardian.com, (June 2018),“Healthcare Security, Understanding HIPPA Compliance, and Its Role in Patient Data Protection”, https://digitalguardian.com/blog/healthcare-security-understanding-hipaa-compliance-and-its-role-patient-data-protection
[15] Editor, CysticFibrosisNewsToday, “Life Expectancy”, https://cysticfibrosisnewstoday.com/cystic-fibrosis-life-expectancy/
[16] Ellingford, Jamie; Beaman, Glenda; Webb, Kevin; O’Callaghan Christopher; Hirst, Robert; on behalf of the 100,000 Genomes Project, Black, Graeme; Newman, William, bioRxiv (October 2018), “Whole genome sequencing enables definitive diagnosis of Cystic Fibrosis and Primary Ciliary Dyskinesia”,
https://www.biorxiv.org/content/biorxiv/early/2018/10/10/438838.full.pdf
[17] Hoffman-Andrews, Lily, Journal of Law and the Biosciences, Volume 4, Issue 3, Pages 648–657, (December 2017), “The known unknown: the challenges of genetic variants of uncertain significance in clinical practice”, https://academic.oup.com/jlb/article/4/3/648/4820755
[18] Weintraub, Karen, Scientific American, (August 2018), “Game changer for cystic fibrosis? Discovery of new cell could alter quest for treatment”, https://geneticliteracyproject.org/2018/08/13/game-changer-for-cystic-fibrosis-discovery-of-new-cell-could-alter-quest-for-treatment/
[19] Raghupathi W, Raghupathi V., PubMed,(Feb 2014),“Big data analytics in healthcare: promise and potential“, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4341817/
[20] Commissioner, US Food and Drug Administration, “Digital Health”,(March 2019)https://www.fda.gov/medical-devices/digital-health
[21] Malokoff, David, Service, Robert F., Science Magazine,” (Feb 2001), Genomania Meets the Bottom Line”, https://science.sciencemag.org/content/291/5507/1193
[22] Ventner, Craig, Patrinos, Ari, Collins, Francis, Web.gov Human Genome News, (2003) “On the Shoulders of Giants, Private Sector Leverages HGP Successes” https://web.ornl.gov/sci/techresources/Human_Genome/project/privatesector.shtml
[23] St Clair, Monica, Mayo Clinic, (Jan 2019) “An Ear for the Unsaid: The Importance of Hearing What is Not Said by Patients”, https://socialmedia.mayoclinic.org/2019/01/18/an-ear-for-the-unsaid-the-importance-of-hearing-what-is-not-said-by-patients
[24] Lugova, Angelina, Quora.com (October 2018), https://www.quora.com/Do-certain-mobile-apps-sell-data-they-take-from-users-phones
[25]Harris, Richard, NPR, (November 2018), “Startup (Nebula Genomics) Offers To Sequence Your Genome Free Of Charge, Then Let You Profit From It”, https://www.npr.org/sections/health-shots/2018/11/15/667946213/startup-offers-to-sequence-your-genome-free-of-charge-then-let-you-profit-from-i
[26]Collins, Francis, NIH, (Aug 2013), “The HeLa Genome: An Agreement on Privacy and Access”, https://www.nih.gov/about-nih/who-we-are/nih-director/statements/hela-genome-agreement-privacy-access
[27] Hudson, Kathy, Collins, Francis, Nature, (Aug 2013), “Family Matters, Henrietta Lacks”, https://www.nature.com/articles/500141a
[28] Administration Staff, US Food and Drug, (September 2018), Mobile Medical Applications, https://www.fda.gov/medical-devices/digital-health/mobile-medical-applications
Jeanne Barnett, [i] Corey Nislow, [ii]
[i] Barnett, Jeanne, Patient Advocate, Teacher, Writer, CEO/Founder CysticFibrosis.com (1996), /CFTechnology.org 501 (c)(3) 2011, User Profile, LinkedIn, viewed 19, April 2019,
CysticFibrosis.com/CFTechnology.org 501 (c)(3); 194 Second Ave, Cedar Grove, NJ 07009, USA,
https://www.linkedin.com/in/jeannebarnett/
[ii] Nislow, Corey, Ph.D,Tier 1 CRC Chair in Translational Genomics, University of British Columbia
6619-2405 Wesbrook Mall,Vancouver, BC, V6T 1Z3, CANADA, http://chemogenomics.pharmacy.ubc.ca