Outcomes and Tolerances in Cystic Fibrosis Treatment Lumacaftor/Ivacaftor
Last month, the results of a study were published that looked at the progress and side effects of the cystic fibrosis treatment lumacaftor/ivacaftor in 116 people with CF during a year after starting the medication.
- 46 (39.7%) people reported side effects related to lumacaftor/ivacaftor, and of the side effects, 82.2% were pulmonary.
- 20 (17.2%) people discontinued the medication because of side effects.
- The mean change in FEV1% predicted was 0.11% (the range was from -39% to +20%).
- 19 people (of the 116 followed) had an FEV1% predicted ≤ 40% prior to treatment, and a higher percentage of this subgroup reported side effects (57.9%, compared to 39.7% of overall group) and a higher percentage of these patients discontinued lumacaftor/ivacaftor (31.6%, compared to 17.2% of the overall group).
- Female gender was associated with a higher odds of drug discontinuation (adjusted odds ratio=3.12, 95% CI, 1.04-9.38).
To summarize, almost 40% of the 116 patients being followed by the study reported side effects. The people starting the medication with ≤40 FEV1 % predicted were more likely to experience side effects and stop the medication due to the side effects.
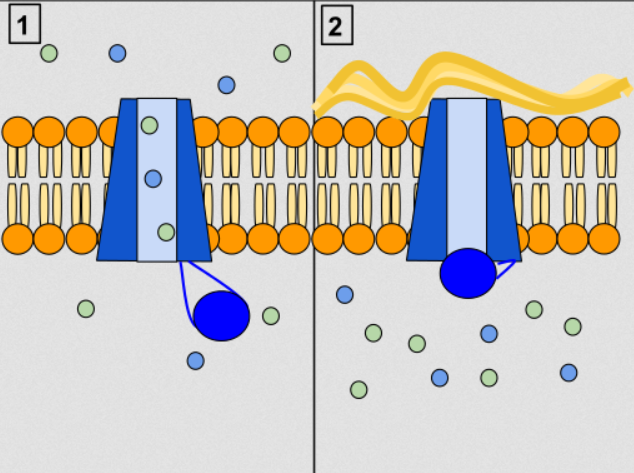
Lumacaftor/ivacaftor targets the main defect in the CFTR protein in people with the F508del mutation. Ivacaftor increases the activity of the CFTR protein at the surface of epithelial cell, while lumacaftor acts as a chaperone during protein folding and increases the number of CFTR proteins that are trafficked to the cell surface (Wikipedia).