New project with Vaultt
We want to introduce you to an exciting new project we have launched at CysticFibrosis.com. After years of effort, we are […]
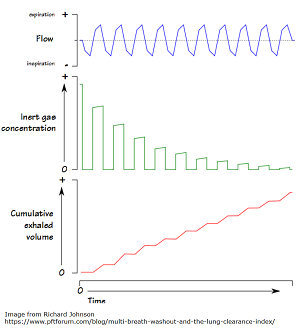
Lung Clearance Index Improved in Children Ages 6-11 on Ivacaftor/Lumacaftor
Ivacaftor/Lumacaftor Improves Lung Clearance Index Score In Children A new phase 3 study, led by The Hospital for Sick Children […]
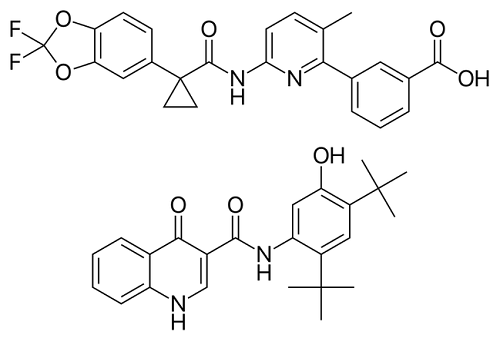
Orkambi Study On Children Age 6-12 With DDF508: Cystic Fibrosis Treatment
Results are in from a 24-week study of Orkambi (lumacaftor/ivacaftor – a medication used in cystic fibrosis treatment) in 58 children […]
Lumacaftor/Ivacaftor longer trial on Heterozygous F508del Cystic Fibrosis Patients
At the end of November, the results of a clinical trial of Lumacaftor/Ivacaftor, also known as Orkambi, in CF patients […]
Drug Development Pipeline for Cystic Fibrosis treatment
The CFF has a interesting and easy to use Drug Development Pipeline webpage which shows the stages of medication that […]
CRISPR Finds Mutated DNA and Is Okayed for Clinical Trial
Since 2003, when we first started saving all our messages here at CysticFibrosis.com, Cystic Fibrosis genetics and our mutations have […]
Matching Volunteers to Clinical Trials by App
It takes on average 17 years for clinical breakthroughs in the laboratory to reach patients. A big part of […]
In the News: Inhaled Levofloxacin & QIDP Designation
Raptor Pharmaceutical’s MP-376 (Inhaled Levofloxacin) granted QIDP Status by the FDA on March 16, 2016. What is Qualified Infectious Disease […]
We Lobby
A Brief History of Our Lobbying Efforts in 2015 In April, 2015, we sent this letter to the FDA […]